Efgartigimod (formerly ARGX-113) Development
Mechanism of Action
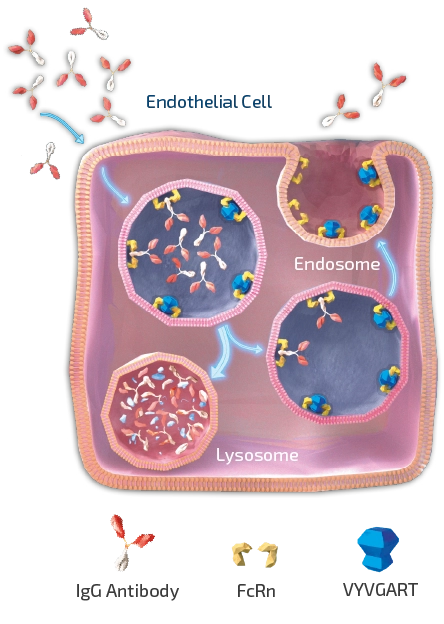
As shown in Figure 1, efgartigimod is a human IgG1 Fc fragment equipped with our ABDEG™ mutations that is designed to target the FcRn and reduce IgG. FcRn is foundational to the immune system and functions to recycle IgG, extending its serum half-life over other Igs that are not recycled by FcRn. IgGs that bind to FcRn are rescued from lysosomal degradation. By binding to FcRn, efgartigimod can reduce IgG recycling and increase IgG degradation.
Compared to alternative immunosuppressive approaches, such as B-lymphocyte (B-cell) depleting agents, efgartigimod acts in a highly selective manner. For efgartigimod, we now have an estimated 4,000 patients years of safety follow-up between clinical trials and real world experience. Efgartigimod has been observed to significantly reduce concentrations of all IgG subtypes without decreasing levels of other Igs or human serum albumin, which is also recycled by FcRn, discussed in more detail in the paragraph of this section on formulations below.
Based on its mechanism of action in targeting FcRn to selectively reducing IgGs, efgartigimod has the potential to address a multitude of severe autoimmune diseases where pathogenic IgGs are believed to be mediators of disease.
As of the end of 2023, we are evaluating efgartigimod in more than 10 serious autoimmune indications. We plan to expand efgartigimod into new indications and plan to be in 15 indications by 2025.
Indication Selection Strategy
We utilize the following strategy to select indications for efgartigimod:
- We first start with a strong, unifying biological rationale. The indications in our pipeline are unified in that there exists a wide range of supportive evidence that demonstrates that each is IgG-mediated. This ranges from published literature, clinical trials with currently used therapies such as IVIg, PLEX, or Rituximab, and other experiments, such as passive transfer models.
- We also look at indications where a significant clinical or commercial opportunity exists. These are disease areas where there is a significant unmet need for innovation as patients are often not well-managed by current therapies and their respective side effects.
- Furthermore, for each indication, there is a defined path forward with established precedent for how to run POC and registrational clinical trials with generally accepted clinical and regulatory endpoints.
- Finally, as we work towards achieving our ‘argenx 2025’ vision, we select indications where there is a reasonable fit within our growing commercial activities.
Formulations
Overview
We are developing two formulations of efgartigimod to address the needs of patients, physicians, and payors across indications and geographies, including IV efgartigimod (VYVGART) and SC efgartigimod (VYVGART SC).
IV (VYVGART)
We conducted a Phase 1 clinical trial in healthy volunteers to evaluate the safety, tolerability, pharmacokinetic (PK), pharmacodynamic (PD), and immunogenicity of single and multiple doses of efgartigimod. In the first part of the clinical trial, 30 subjects were randomized to receive a single dose of efgartigimod or placebo ranging from 0.2 mg/kg to 50 mg/kg. In the second part of the clinical trial, 32 subjects were randomized to receive multiple ascending doses (MADs) of efgartigimod or placebo up to a maximum of 25 mg/kg.
In the MAD part of the Phase 1 clinical trial, repeat administration of both 10 mg/kg and 25 mg/kg of efgartigimod every seven days, four doses in total, and 10 mg/kg every four days, six doses in total, was associated with a gradual reduction in levels of all four classes of IgG antibodies by 60% to 85%, with 10 mg/kg dose results shown in Figure 2. For all doses in the MAD part of the Phase 1 clinical trial, we observed the reduction in circulating IgG antibody levels to persist for more than four weeks after the last dose with levels below 50% at approximately three weeks and did not return to baseline levels for more than one month. PK analysis of serum baseline levels of efgartigimod indicates that it has a half-life of approximately three to four days with no drug accumulation following subsequent weekly dosing. The prolonged activity on the levels of IgG antibodies is consistent with the mechanism of action of efgartigimod and the effect of our proprietary ABDEG™ technology (detailed in section “Platform Technologies”) on increasing the intracellular recycling of efgartigimod. In both the single and MAD portions, no significant reductions in immunoglobulin M (IgM), immunoglobulin A (IgA) or serum albumin were observed.
SC (VYVGART SC) – Partnership with Halozyme
In July 2019, we evaluated a first generation of SC efgartigimod that incorporates Halozyme’s ENHANZE® SC drug delivery technology in a Phase 1 clinical trial in healthy volunteers, which demonstrated retained PD profile of IV efgartigimod.
ENHANZE® has demonstrated across multiple FDA-approved products the ability to remove traditional limitations on the volume of biologics that can be delivered subcutaneously, potentially shortening drug administration time, reducing healthcare practitioner time, and offering additional flexibility and convenience for patients.
In 2020, we expanded the existing global collaboration and license agreement with Halozyme. Under the expansion, we gained the ability to access Halozyme’s ENHANZE® SC drug delivery technology for three additional exclusive targets upon nomination bringing the total to six potential targets under the collaboration. To date, two targets have been nominated including FcRn and C2.
In March 2022, we announced our Phase 3 ADAPT-SC clinical trial evaluating SC efgartigimod achieved the primary endpoint of total IgG reduction from baseline at day 29, demonstrating statistical non-inferiority to VYVGART IV formulation in gMG patients. Based on these results, we received approval of VYVGART SC for the treatment of adult patients with gMG in the U.S., the EU, the UK and Japan.
Currently, we are developing a pre-filled syringe presentation for the same SC formulation using the Halozyme technology, to allow for a convenient delivery and the potential for self-administration, reducing the healthcare practitioner time and further increasing flexibility and convenience for patients. As a next step in patient convenience, we have also started the development of a high-volume auto-injector.
SC – Partnership with Elektrofi
In April 2021, we entered into a collaboration and license agreement with Elektrofi to explore a high concentration technology for efgartigimod and up to one additional target. Please refer to “Our Exclusive License with Elektrofi for efgartigimod” for more information.
