efgartigimod (formerly ARGX-113) Development
Mechanism of Action
As shown in Figure 1, efgartigimod is a human IgG1 Fc fragment equipped with our ABDEG™ mutations that is designed to target the FcRn and reduce IgG. FcRn is foundational to the immune system and functions to recycle IgG, extending its serum half-life over other IgGs that are not recycled by FcRn. IgGs that bind to FcRn are rescued from lysosomal degradation. By binding to FcRn, efgartigimod can reduce IgG recycling and increase IgG degradation.
Compared to alternative immunosuppressive approaches, such as B-lymphocyte (B-cell) depleting agents, efgartigimod acts in a highly selective manner. For efgartigimod, we now have an estimated 8,000 patients years of safety follow-up between clinical trials and real world experience. efgartigimod has been observed to significantly reduce concentrations of all IgG subtypes without decreasing levels of other IgGs or human serum albumin, which is also recycled by FcRn, discussed in more detail in the paragraph of this section on formulations below.
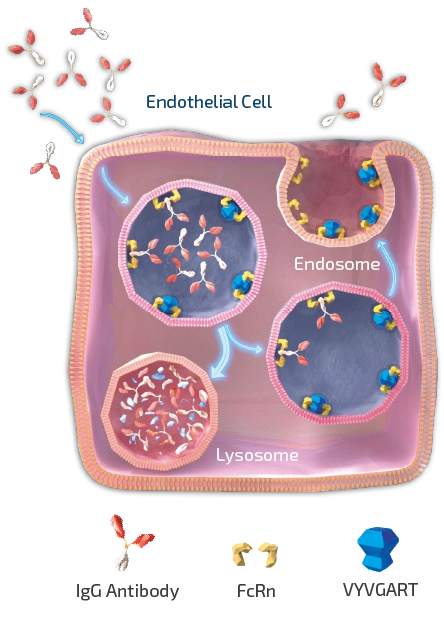
Based on its mechanism of action in targeting FcRn to selectively reducing IgGs, efgartigimod has the potential to address a multitude of severe autoimmune diseases where pathogenic IgGs are believed to be mediators of disease.
As of the end of 2024, we are evaluating efgartigimod in more than 10 serious autoimmune indications and plan to continue to expand into new indications.
Indication Selection Strategy
We utilize the following strategy to select indications for efgartigimod:
- We first start with a strong, unifying biological rationale. The indications in our pipeline are unified in that there exists a wide range of supportive evidence that demonstrates that each is IgG-mediated. This ranges from published literature, clinical trials with currently used therapies such as IVIg, PLEX, or rituximab, and other experiments, such as passive transfer models.
- We also look at indications where a significant clinical or commercial opportunity exists. These are disease areas where there is a significant unmet need for innovation as patients are often not well-managed by current therapies and their respective side effects.
- Furthermore, for each indication, there is a defined path forward with established precedent for how to run POC and registrational clinical trials with generally accepted clinical and regulatory endpoints.
Formulations
Overview
We are developing two formulations of efgartigimod to address the needs of patients, physicians, and payers across indications and geographies, including efgartigimod IV (VYVGART) and efgartigimod SC (VYVGART SC).
Scientific Publications
We refer to our key scientific publications from our Phase 3 studies with either the IV or SC formulation in gMG, ITP and CIDP.
- Publication in The Lancet Neurology of Phase 3 ADAPT study data in generalized myasthenia gravis: thelancet.com/journals/laneur/article/PIIS1474-4422(21)00159-9/abstract
- Publication in The Lancet of Phase 3 ADVANCE-IV study data in primary immune thrombocytopenia: thelancet.com/journals/lancet/article/PIIS0140-6736(23)01460-5/abstract
- Publication in The Lancet Neurology of Phase 3 ADHERE study data in chronic inflammatory demyelinating polyneuropathy: thelancet.com/journals/laneur/article/PIIS1474-4422(24)00309-0/abstract
